3D Printing for Personalized Medicine and Customized Drug Delivery - 3D-CURE
3D printing has been recognized as a potential production technology in the pharmaceutical industry as well. 3D printing enables multi-component materials and complex geometries that are difficult to manufacture with conventional manufacturing methods. Utilizing these properties improves the effectiveness of one-size-fits-all drugs and allows for tailored medication based on the patient's condition for short- or long-term use.
Despite these possibilities, there are several challenges associated with the broader industrial-scale adoption of 3D printing. Firstly, the adaptation of various biomaterials for 3D printing of controlled drug delivery devices is still in its infancy, and there are many drug molecules for which there is no proper solution for 3D printing yet. Even encapsulating traditional small molecule drugs into 3D prints is challenging, but encapsulating biological drug molecules is particularly demanding, as they do not tolerate high processing temperatures.
Thus, designing and adapting new materials for encapsulating drugs into 3D prints is an essential research direction to address these challenges. To ensure safe and efficient production, the entire drug delivery device production process, from design to manufacturing, needs to be digitized so that the flexibility offered by 3D printing technologies can be fully utilized in the pharmaceutical industry. This, in turn, requires the development of new computational models and digital twins as well as in-process quality control.
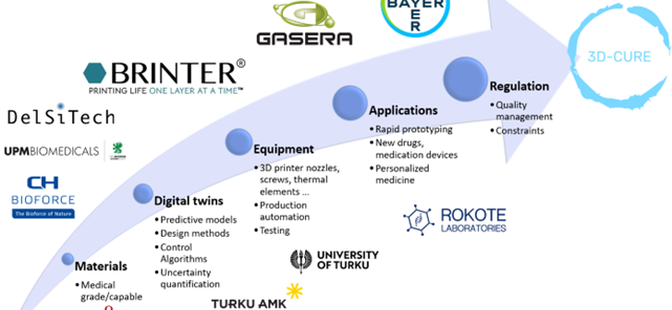
The 3D-CURE project brings together four different research organizations and several companies. The project is led by Turku University of Applied Sciences while Åbo Akademi, Turku University and Lappeenranta-Lahti University of Technology, together with Bayer, Brinter, Gasera, CH-BioForce, UPM Biomedicals, Rokote Laboratories, DelsiTech, and EDR & Medeso form a consortium, whose goal is to develop an entirely new way of designing individual medication on a data and model basis, as well as productionalization of the process through 3D printing testing of different materials.
The consortium covers the entire value chain from raw materials to the design of new drugs and multi-component drug delivery devices, manufacturing using 3D printing, and quality management. In addition, the project tracks regulations to anticipate future research and development regulations for 3D-printed drugs. The project hopes to generate new knowledge of the suitability of 3D printing for drug manufacturing and to explore possibilities for real-time monitoring of the 3D printing process.
Key objectives of the project:
- Testing and development of new (multi-component) medical-grade materials, formulation processes and products specifically tailored for 3D printing
- Digitalization of the drug development process by accurate numerical models of material transport and 3DP production, addressing quality control and uncertainty
- Definition and testing of critical material attributes and critical process parameters to identify critical quality attributes for the 3DP products and define their quality target product profiles
- Analyzing and following the regulatory landscape for 3DP medicinal products to anticipate future regulations on research and product development of 3DP medicinal products
- Testing and development of Photoacoustic Spectroscopy (PAS) for online process monitoring to validate (and eventually control in near real-time) the end-product quality.

